To determine mutual solubility curve for phenol and water
Introduction
Oil and water
don’t mix. Pouring 10 ml of olive oil into 10 ml of water results in two
distinct layers, clearly separated by a curved meniscus. Each layer has the same volume and essentially the same composition as the original
liquids. Because
very little mixing has apparently occurred, the liquids are called “immiscible” or unmixable.
Pouring grain
alcohol into water results in a single liquid phase. No meniscus forms between the alcohol and the water, and
the two liquids are considered “miscible”.
Nearly any pair of liquids is miscible if only a trace amount of one of the
liquids is present.
Many liquid
mixtures fall between these two extremes. Two liquids are “partially miscible” if shaking equal volumes of the liquids together
results in a meniscus visible between two layers of liquid, but the
volumes of
the layers are not identical to the volumes of the liquids originally mixed. For example, shaking water with certain
organic acids results in two clearly separate layers, but each layer contains
water and acid (with one layer mostly water and the other, rich in acid.)
Liquids tend to be immiscible when attractions between like molecules are much stronger than attractions between
mixed
pairs. (Logan, 1998)
Chemicals
 |
| Phenol |
 |
| Distilled water |
Apparatus
 |
| Water Bath |
 |
| Thermometer |
 |
| Test Tubes |
 |
| Measuring cylinder |
Experimental Procedures
1. Tightly sealed tubes containing
amounts of phenol and water to produce different
phenol
concentrations were prepared.
concentrations were prepared.
4. The average temperature of the two
readings was determined. Some of the tubes were cooled rather than heated.
Results
% by Concentration of Phenol
|
Volume of phenol (mL)
|
Volume of water (mL)
|
Temperature (°C) |
||
When the liquid turns clear
|
Average Temperature
|
||||
8.0
|
1.6
|
18.4
|
70.0
|
35.0
|
52.5
|
11.0
|
2.2
|
17.8
|
59.0
|
41.0
|
50.0
|
20.0
|
4.0
|
16.0
|
69.0
|
66.0
|
67.5
|
30.0
|
6.0
|
14.0
|
73.0
|
71.5
|
72.3
|
50.0
|
10.0
|
10.0
|
69.0
|
66.0
|
67.5
|
63.0
|
12.6
|
7.4
|
66.0
|
59.0
|
63.0
|
70.0
|
14.0
|
6.0
|
75.0
|
41.0
|
58.0
|
80.0
|
16.0
|
4.0
|
59.0
|
35.0
|
47.0
|
Discussion
In this experiment we are about to observe two component system
containing liquid phases. As we know from that ethyl alcohol (ethanol) and
water are miscible liquid in all proportions, whereas water and mercury are,
for all practical purposes, completely immiscible regardless of the relative
amounts of each present. In this experiment, we will use such a system
involving phenol and water in which the phenol is not really liquid, but is
considered to be so since the addition of the first part of water reduces the
solid’s melting point under room temperature to produce a liquid-liquid system.
Upper diagram shows the theoretical phase diagram where we should get during the
plotting the result of the experiment. Let us explain first about the
theoretical phase diagram on the left before we compare with our result. Note
that the region inside the curve shows the limit of the temperature and
concentration within which two liquid phases exist in equilibrium. While the
region outside of this curve contains systems having only one liquid phase. The
addition of known increments of phenol to a fixed weight of water, the whole
being maintained at 50°C, will result in the formation of a single liquid phase
until the point (~11% of phenol, 50°C) is reached, at which a minute amount of
a second phase appears. The phenol rich phase is denoted by the point (~63% of
phenol, 50 °C) on the phase diagram. Once the total concentration of phenol
exceeds 63%, at 50°C, a single phenol rich phase is formed. The maximum
temperature at which the two phase region exists is termed as the ‘critical
temperature or upper consolute temperature’ which theoretically at 66.8°C. t.
Above the critical temperature, phenol and water are completely miscible. For
the ‘tie line’, it is always parallel to the base line in two component
systems. The tie line at equilibrium will separate into phases of constant
composition which is called as ‘conjugate phases’. Now let us compare our results with the
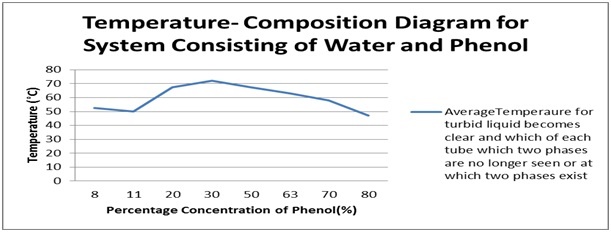
theory, our phase diagram is shown below:
If we look at our phase diagram, we can see that it is slightly differ but not too much when we compare with the theoretical phase diagram. The difference may cause by some errors during conducting the experiment. The starting temperature for 8% concentration of phenol should be lower than the temperature at 11% concentration of phenol, but due to the errors during the experiment, we’re not getting the accurate result that follow the theory. Moreover, from the graph we also can see that our critical temperature is at 72.3°C which a little bit increase than the theory that is 66.8°C. Once again, this might due to the presence of error during the experiment. Note that the region inside the graph shows that phenol and water is immiscible and form two layers of liquid, while the region outside the graph or beyond the critical temperature shows that the mixture of phenol and water is miscible and form single layer of liquid. Overall, we can say that our shape of the graph had followed the shape of the theory’s graph. The examples of errors that had present during the experiment are:
1)
Parallax
error in which our eyes are not perpendicular to the reading scale of the
measuring cylinder during measuring the liquid of phenol and water. We should make sure that our eyes is always
perpendicular to the reading scale to ensure a precise and correct measurement
in order to get a better result in the future.
2)
Not
shaking the mixture well. This causes the rate of turbidity of solution to
become clear to be longer and slower, thus it will affect the accuracy of the
temperature for each tube at which two phases to become one phase. We must make
sure that we shake the tube very well in the water bath so that an equilibrium
heat can be supply in order to turn the turbid mixture into a clear mixture.
3)
Not
sealing the tube well with rubber stopper. This may cause faster losses of heat
to the surrounding and we may not get the correct temperature for the mixture
to be cooled and formed two phase of liquid back. Thus, we must make sure that
we sealed the mouth of the test tube tightly to get a better quality of
results.
Questions
1. Discuss the
diagram with reference to the phase rule.
The diagram is a two component condensed system having
one liquid phase since phenol and water are miscible with each other at a
particular condition.
Based on the formula, F=C-P+2
F is degree of
freedom
C is numbers
of component
P is number of
phase exist
Therefore the
degree of freedom, F = 2 − 1 + 2 = 3. The pressure is fixed for this system,
thus F is reduced to 2. From the diagram, if the temperature is given, the
composition of the mixture can be determined easily through the diagram. In
short, only two independent variables are required to define the phenol and
water system completely.
2. Explain the
effect of adding foreign substances and the importance of this effect in pharmaceutical .
Solubility of
liquids in liquids is very important in preparation of drug in pharmacy. It is
very common for two or more liquids to be mixed together in a pharmacy to make
a solution, therefore the pharmacist needs to know what liquids can be mixed
together without precipitation occurring. The influence of a foreign substance
on a liquid-liquid system is similar to the idea of a three component system in
the phase rule we studied earlier. The degree of freedom and miscibility of the
two liquid will be affected. If the contaminant reduces the miscibility of the
two liquid, the dispensed medicine may changes its nature and no longer
suitable for consumption. Besides, the therapeutic effect of some drug will be
reduced and may be harmful to human body. This condition may be arising due to
contamination in extemporaneous preparation when the place of medicine
preparation is not hygienic.
Conclusion
Based on our experiment we can say that we had achieved the theory as we
learned how to plot the phase diagram graph and get a better understanding
about the theory regarding the phase diagram that involve two component system.
1)http://books.google.com.my/books? id=NFGSSSbaWjwC&pg=PA217&lpg=PA217&dq=mutual+solubility+curve+for+phenol+and+water&source=bl&ots=V71YTihssv&sig=NH5STbhBl7OmVpu_OE0jj63HFZI&hl=en&sa=X&ei=5UyoUaaNDIKyrgeUkICgDg&ved=0CC4Q6AEwAA#v=onepage&q=mutual%20solubility%20curve%20for%20phenol%20and%20water&f=false
2)http://www.d.umn.edu/~psiders/courses/chem4643/labinstructions/phenol.pdf
3)http://books.google.com.my/books?
id=FSW3rUCHfEMC&pg=PA41&lpg=PA41&dq=mutual+solubility+curve+for+phenol+and+water&source=bl&ots=UhLyrxGllx&sig=d3VwBDe5BZ91oRAG1sAuHqg3wKs&hl=en&sa=X&ei=5UyoUaaNDIKyrgeUkICgDg&ved=0CEkQ6AEwBg#v=onepage&q=mutual%20solubility%20curve%20for%20phenol%20and%20water&f=false
2)http://www.d.umn.edu/~psiders/courses/chem4643/labinstructions/phenol.pdf
3)http://books.google.com.my/books?
id=FSW3rUCHfEMC&pg=PA41&lpg=PA41&dq=mutual+solubility+curve+for+phenol+and+water&source=bl&ots=UhLyrxGllx&sig=d3VwBDe5BZ91oRAG1sAuHqg3wKs&hl=en&sa=X&ei=5UyoUaaNDIKyrgeUkICgDg&ved=0CEkQ6AEwBg#v=onepage&q=mutual%20solubility%20curve%20for%20phenol%20and%20water&f=false
4) Christopher J. Moody. 1999. Experimental Chemistry: Principles and Practice. Illustrated edition. Page 118-122. New York: Blackwell.
5) Benjamin Abelow. 1998. Physical Pharmacy Principles. 1st edition. Page 97-
103. United States: Library of Congress.





These are pipettes to measure micro liter of volume. Sales of Test & Measurement Assets
ReplyDelete